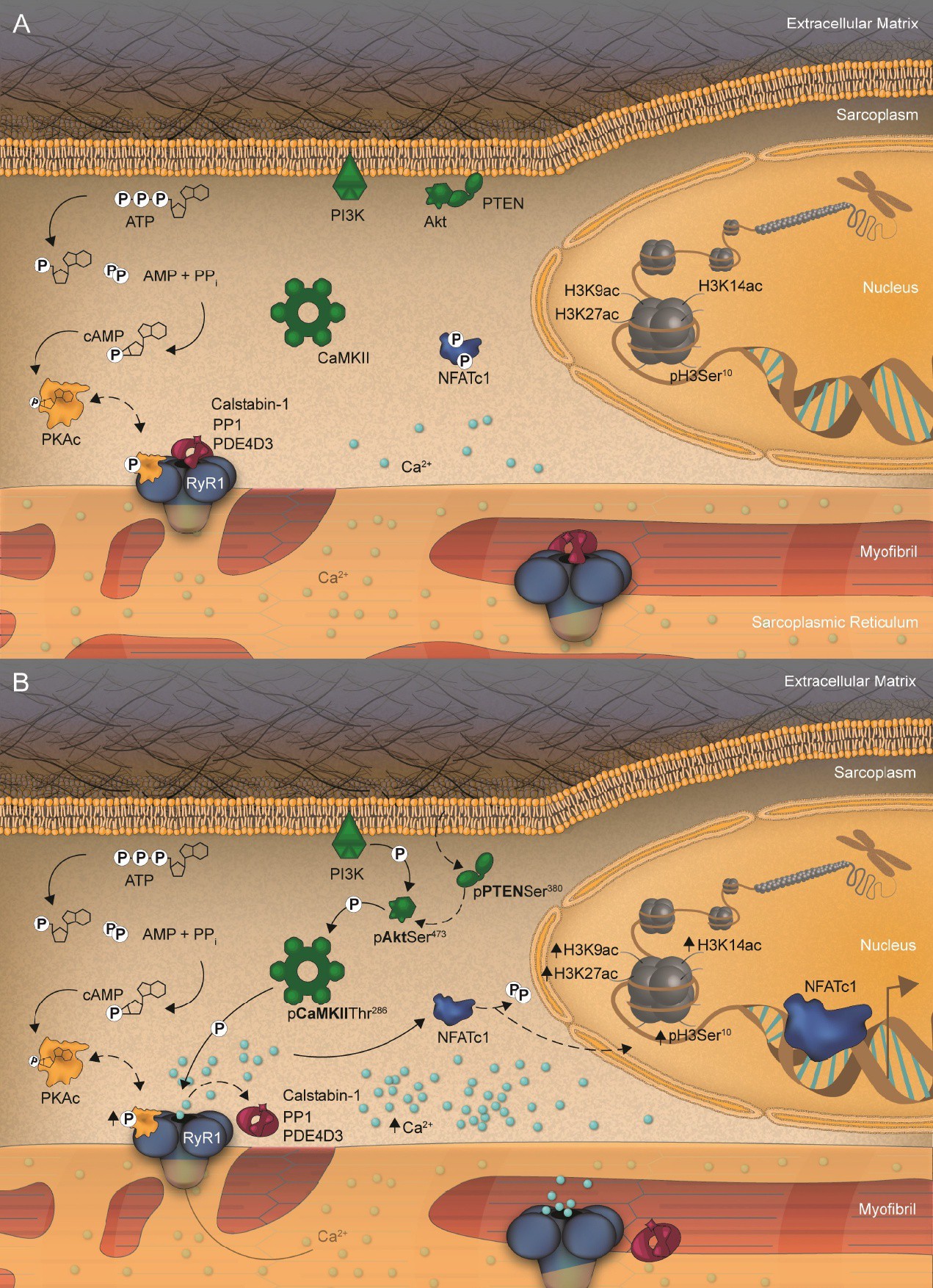
Fig. 6. Summary of RyR1 phosphorylation-triggered signaling and mediated NFATc1 and epigenetic histone H3 modifications induced by acute exercise. (A) Under resting conditions, the ATP metabolism is low as is the cAMP concentration. Therefore, only small amounts of the apoenzyme PKA are cleaved into its active catalytic isoform, PKAc. Subsequently, RyR1 phosphorylation is low, but not absent, and the RyR1 stabilizing molecules, calstabin-1, PDE4D3, and PP1, are associated to RyR1. In parallel, resting conditions result in low inactive (phosphorylated) forms of PTEN. Likewise, there is little activation of Akt at Ser473 and, thus, of CaMKII at Thr286. This results in the regular oscillation of Ca2+ from RyR1, similar to what occurs in the event of low PKAc. The consequences are sarcoplasmic NFATc1 localizations as well as low epigenetic H3 acetylations/phosphorylations. (B) Acute in vivo exercise induces high ATP metabolism in working skeletal muscles resulting in increased cAMP levels. This event activates the apoenzyme PKA to be cleaved into its catalytic isoform, PKAc. Subsequently, RyR1 phosphorylation increases and its stabilizing molecules, calstabin-1, PDE4D3, and PP1, show transient dissociations from RyR1. In parallel, acute exercise leads to increased levels of the inactive (phosphorylated) form of PTEN, enabling Akt to be PI3K-phosphorylated at Ser473 (bold). Subsequently, CaMKII is activated at Thr286, which might amplify the PKAc-mediated RyR1 phosphorylation. Consequently, NFATc1 is dephosphorylated and translocates into the nucleus and H3 is epigenetically modified by specific acetylations. Synergistically, these events control skeletal muscle properties and molecular phenotypes even under conditions of acute and short-term muscle work.